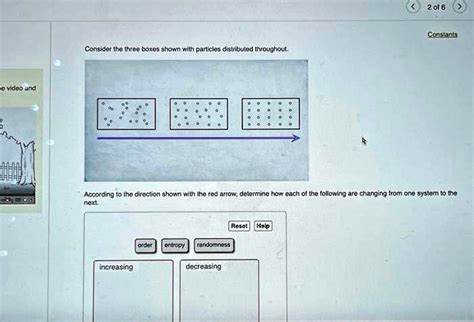
consider the three boxes shown with particles distributed throughout. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table .
Local industrial welding and metal fabrication services to fit any need. Certified and insured welders with over a decade of experience.Look to SLS Metalworks and Fabricating for Custom Welding, Repairs, Structural Steel Fabrication, Brake & Shear, Aluminium Welding, Mobile welding, galvanizing, powder coating .
0 · VIDEO solution: 2 of 6 Constants Consider the three boxes
1 · U4 Hw3: density Flashcards
2 · Solved Select Play to watch the video on the second law of
3 · Solved Consider the three boxes shown with particles
4 · SOLVED: Consider the three boxes shown with particles
5 · Figure 1 B FIGURE 1 Worksheet Density A B Comparison of
6 · Entropy and a box of air
7 · Consider the three boxes shown with particles distributed
$42.99
Consider the three boxes shown with particles distributed throughout. Your solution’s ready to go! Enhanced with AI, our expert help has broken down your problem into an easy-to-learn . Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level . Study the matter shown in Figure 1. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] How do the masses of A .
As we consider boxes with more particles, the preference for an even distribution gets much much stronger as the number of microstates in a macrostate explodes. For example, suppose we have a box with 1000 molecules.
2 of 6 Constants Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the .Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table . Step 1: In the first system, the particles are evenly distributed throughout the box. Step 2: In the second system, the particles are moving towards the right, following the .
Consider the three boxes shown with particles distributed throughout. [: [00000000000000000000]}According to the direction shown with the red arrow, determine how .Consider the three boxes shown with particles distributed throughout. Each box from left to right has 2 0 particles inside. The particles in the first box are scattered around with no apparent .Question: Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next.Consider the three boxes shown with particles distributed throughout. Your solution’s ready to go! Enhanced with AI, our expert help has broken down your problem into an easy-to-learn solution you can count on.
Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level of order, as the particles are evenly distributed.
VIDEO solution: 2 of 6 Constants Consider the three boxes
Study the matter shown in Figure 1. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] How do the masses of A and B compare? *Mass A > Mass B *Mass A < Mass B *Mass A = Mass BAs we consider boxes with more particles, the preference for an even distribution gets much much stronger as the number of microstates in a macrostate explodes. For example, suppose we have a box with 1000 molecules.
conduit pvc electrical box single
2 of 6 Constants Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next: Entropy Randomness increasing decreasingEach dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table below, show how the masses, volumes, and densities of A and B compare by adding the symbol <, >, or = to the statement in the second column. Step 1: In the first system, the particles are evenly distributed throughout the box. Step 2: In the second system, the particles are moving towards the right, following the direction of the red arrow.
Consider the three boxes shown with particles distributed throughout. [: [00000000000000000000]}According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next. Your solution’s ready to go!Consider the three boxes shown with particles distributed throughout. Each box from left to right has 2 0 particles inside. The particles in the first box are scattered around with no apparent pattern. The particles in the second box are close to forming a grid that is four particles high and five particles long.Question: Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next.
U4 Hw3: density Flashcards
Consider the three boxes shown with particles distributed throughout. Your solution’s ready to go! Enhanced with AI, our expert help has broken down your problem into an easy-to-learn solution you can count on. Step 1: In the initial system, the particles are distributed evenly in the three boxes, with two particles in each box. This arrangement can be considered as having a certain level of order, as the particles are evenly distributed.
Study the matter shown in Figure 1. Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] How do the masses of A and B compare? *Mass A > Mass B *Mass A < Mass B *Mass A = Mass BAs we consider boxes with more particles, the preference for an even distribution gets much much stronger as the number of microstates in a macrostate explodes. For example, suppose we have a box with 1000 molecules.
2 of 6 Constants Consider the three boxes shown with particles distributed throughout. According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next: Entropy Randomness increasing decreasing
Each dot represents a particle of matter. [Assume the particles are uniformly distributed throughout each object, and particles of the same size have the same mass.] In the table below, show how the masses, volumes, and densities of A and B compare by adding the symbol <, >, or = to the statement in the second column. Step 1: In the first system, the particles are evenly distributed throughout the box. Step 2: In the second system, the particles are moving towards the right, following the direction of the red arrow.

Consider the three boxes shown with particles distributed throughout. [: [00000000000000000000]}According to the direction shown with the red arrow, determine how each of the following are changing from one system to the next. Your solution’s ready to go!
Solved Select Play to watch the video on the second law of

condulet junction box
concrete cnc milling machine
To weld thin metal, choose an appropriate process (TIG or MIG with low amperage), use a smaller diameter wire or filler rod, and adjust travel speed to prevent burn-through. Employ a pulsing technique, stitch welding, or tack welds to manage heat and allow cooling periods between welds to minimize warping.
consider the three boxes shown with particles distributed throughout.|VIDEO solution: 2 of 6 Constants Consider the three boxes