alkaline earth metals periodic table boxes The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. Together with helium, these elements have in common an outer s orbital which . $163.48
0 · alkaline earth metals shell
1 · alkaline earth metals pdf
2 · alkaline earth metals list
3 · alkaline earth metals group 2
4 · alkaline earth metals diagram
5 · alkaline earth metals chemistry
6 · alkaline earth metal properties
7 · alkaline earth element chart
A junction box provides a safe, code-compliant space for housing cable connections for outlets, switches, or splices. They prevent potential electrical shocks, and keep sparks from spreading to flammable surroundings.
The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Atoms of each of these elements have two electrons in the outer electron shell. Take a look .
stock thickness sheet metal
The alkaline earth elements are in Group 2 of the periodic table. These elements each have two s electrons in their outer shell. The alkaline earth elements are less reactive than the alkali metals.The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. Together with helium, these elements have in common an outer s orbital which .Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2] .
Alkaline Earth Metals are a set of six chemical elements in the periodic table’s group 2. Beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) are the elements involved (Ra). Alkaline .An interactive periodic table featuring elements categorized as Alkaline Earth Metals. The Group 2 metals have a particular name: the alkaline earth metals. The name is derived from the observation that they have such high melting points (Table 4.1.4.1) that they remain solids (earths) in a fire. Table .
These metals are any of the metallic elements within Group 2 in the Periodic Table (see the List of Alkaline metals). The name Alkaline Earth Metals refer to their oxides that simply give basic .
Alkaline Earth Metals Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. Those earths, such as lime .
The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Atoms of each of these elements have two electrons in the outer electron shell. Take a look at the elements in this group and their common properties: List of the Alkaline Earth Metals. There are six alkaline earths.
Alkaline-earth metal, any of the six chemical elements that comprise Group 2 of the periodic table. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The alkaline-earth elements are highly metallic and are good conductors of electricity.The alkaline earth elements are in Group 2 of the periodic table. These elements each have two s electrons in their outer shell. The alkaline earth elements are less reactive than the alkali metals.The alkaline earth metals are six chemical elements in group 2 of the periodic table.They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). [1] The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. [2]Together with helium, these elements have in .
Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2] . Alkaline Earth Metals are a set of six chemical elements in the periodic table’s group 2. Beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) are the elements involved (Ra). Alkaline Earth Metals are s-block elements as the last electron enter into the s-subshell.
An interactive periodic table featuring elements categorized as Alkaline Earth Metals. The Group 2 metals have a particular name: the alkaline earth metals. The name is derived from the observation that they have such high melting points (Table 4.1.4.1) that they remain solids (earths) in a fire. Table 4.1.4.2 lists the derivation of the names of the alkali metals.These metals are any of the metallic elements within Group 2 in the Periodic Table (see the List of Alkaline metals). The name Alkaline Earth Metals refer to their oxides that simply give basic alkaline solutions. Alkaline Earth Metals Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. Those earths, such as lime (calcium oxide), that resembled the alkalies (soda ash and .
The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Atoms of each of these elements have two electrons in the outer electron shell. Take a look at the elements in this group and their common properties: List of the Alkaline Earth Metals. There are six alkaline earths.
Alkaline-earth metal, any of the six chemical elements that comprise Group 2 of the periodic table. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The alkaline-earth elements are highly metallic and are good conductors of electricity.
alkaline earth metals shell
The alkaline earth elements are in Group 2 of the periodic table. These elements each have two s electrons in their outer shell. The alkaline earth elements are less reactive than the alkali metals.The alkaline earth metals are six chemical elements in group 2 of the periodic table.They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). [1] The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. [2]Together with helium, these elements have in .Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2] .
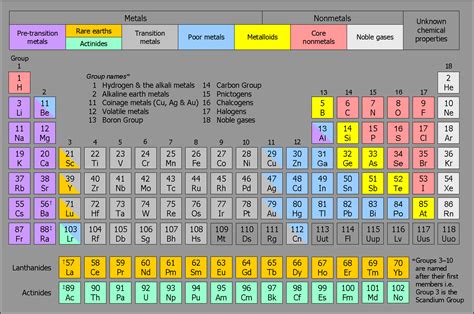
Alkaline Earth Metals are a set of six chemical elements in the periodic table’s group 2. Beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) are the elements involved (Ra). Alkaline Earth Metals are s-block elements as the last electron enter into the s-subshell.An interactive periodic table featuring elements categorized as Alkaline Earth Metals. The Group 2 metals have a particular name: the alkaline earth metals. The name is derived from the observation that they have such high melting points (Table 4.1.4.1) that they remain solids (earths) in a fire. Table 4.1.4.2 lists the derivation of the names of the alkali metals.These metals are any of the metallic elements within Group 2 in the Periodic Table (see the List of Alkaline metals). The name Alkaline Earth Metals refer to their oxides that simply give basic alkaline solutions.
alkaline earth metals pdf
New and used Metal Filing Cabinets for sale in Pretoria, South Africa on Facebook Marketplace. Find great deals and sell your items for free.
alkaline earth metals periodic table boxes|alkaline earth metal properties